
Axonics aspires to be the global leader in incontinence therapies by providing customer-centric solutions to improve the quality of life for patients suffering from urinary and bowel dysfunction.



The Axonics F15 is the Longest-Lived and TRULY Recharge-Free SNM System with a 17.6 year battery life at 1mA and over 20 years at lower energy settings.*
*Depending on therapy settings
Axonics R20 is an easy-to-use system that offers your patients a long-lasting therapy designed to last 20+ years, and only requires recharging every 6 to 10 months.*
*Recharge interval depends on therapy settings


Before Axonics, many of my passions had been sidelined. Embracing my active life again has been a game-changer for me.
Results and experiences may vary and are unique to each patient.

Homogenous hydrogel consisting of 97.5% water and 2.5% cross-linked polyacrylamide1.
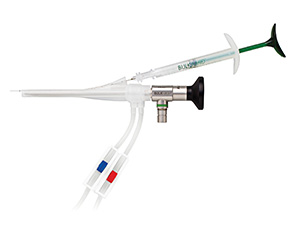
Allows for reproducible placement of the Bulkamid cushions under direct vision.
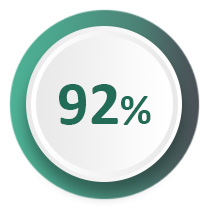
92% of women report being cured or improved at 12 months2, with durability demonstrated at seven years3 and no serious adverse events4.

Before Bulkamid, I couldn't play with my kids without worrying about leaks. But now, I have real relief.
Results and experiences may vary and are unique to each patient.
Provide your information below to be contacted by the Axonics Representative near you.
*Recharge interval depends on therapy settings

Indications:
Axonics SNM Therapy for urinary control is indicated for the treatment of urinary retention and the symptoms of overactive bladder, including urinary urge incontinence (leakage) and significant symptoms of urgency-frequency, either alone or in combination. Axonics SNM Therapy for bowel control is indicated for the treatment of chronic fecal incontinence. The therapy should only be used in patients who have failed or are not candidates or could not tolerate more conservative treatments.
Contraindications:
The Axonics SNM System is contraindicated for patients who have not demonstrated an appropriate response to test stimulation or patients who are unable to operate the Axonics SNM System.
Warnings:
Implantation and use of the Axonics System incurs risk beyond those normally associated with surgery, some of which may necessitate surgical intervention. These risks include, but are not limited to adverse change in voiding function (bowel and/or bladder), infection, pain or irritation at the implant site, lead or device migration, electrical shock, change in sensation or magnitude of stimulation which has been described as uncomfortable (jolting or shocking) by some patients, and heating or burns at the device site.
Precautions:
Implanting clinicians should be trained on the implantation and use of the Axonics SNM Systems. Prescribing clinicians should be experienced in the diagnosis and treatment of lower urinary tract symptoms and should be trained on the use of the Axonics SNM Systems.
The safety and effectiveness of Axonics Therapy has not been established for use in pregnant women, the unborn fetus, and during delivery, for pediatric patients (under the age of 18 years for FI and under the age of 16 years for OAB and UR), for patients with neurological disease origins, such as multiple sclerosis or diabetes, or for bilateral stimulation.
Caution: U.S. Federal law restricts this device to sale and use by, or on the order of, a physician.
For a complete listing of indications, contraindications, warnings and precautions, go to www.axonics.com/isi.

Indications:
The Bulkamid Urethral Bulking System is indicated for urethral injection for the treatment of stress urinary incontinence (SUI) due to intrinsic sphincter deficiency (ISD) in adult women who have SUI or stress predominant mixed incontinence.
Contraindications:
Bulkamid Urethral Bulking System must not be used in patients suffering from acute urinary tract infection.
Warnings:
Do not inject Bulkamid Hydrogel intravascularly. Injection of Bulkamid Hydrogel into blood vessels may cause vascular occlusion leading to a possible embolism. Discontinue injection of Bulkamid Hydrogel if the superficial capillaries of the mucosa start to fade in order to avoid ischemia. Prior assessment of the tissue is recommended before introducing the Bulkamid Rotatable Sheath into the urethra. Do not force the Bulkamid Rotatable Sheath into the urethra or inject Bulkamid Hydrogel if the urethral tissue is damaged. The Bulkamid Urethral Bulking System should not be used in patients with urethral or bladder neck strictures until the strictures have been corrected. Use of the Bulkamid Urethral Bulking System in patients with strictures may cause injury and/or urethral obstruction. Over-correction using Bulkamid Hydrogel may lead to obstruction. Patients receiving treatment affecting blood coagulation have an increased risk of hematoma or urethral bleeding, as with any invasive procedure. Do not use Bulkamid Hydrogel in male patients.
Precautions:
The Bulkamid Urethral Bulking System is only to be administered by a qualified physician, e.g. gynecologist, urologist, or urogynecologist. Safety and effectiveness of Bulkamid have not been established in patients with a fragile urethral mucosal lining, with urethral hypermobility with a straining angle >30º from horizontal bladder neck, predominant urge incontinence, detrusor overactivity, known polyuria (≥ 3L/24h), unevaluated hematuria, prolapse stage greater than Stage II using the ICS Pelvic Organ Prolapse Quantification (POPQ) exam, BMI >35 kg/m2, neurogenic bladder, less than 18 years of age, have active Herpes Genitalis, or for re-injection of Bulkamid Hydrogel less than 4 weeks after initial injection. The effect of Bulkamid has not been evaluated in women during pregnancy, delivery or lactation. The effect of Bulkamid on subsequent pregnancy and delivery, and the impact of subsequent pregnancy on the effect of Bulkamid, is unknown. Therefore, the risks and benefits of the device in women of childbearing potential should be carefully assessed.
Adverse Events:
Adverse events may include: pain at the implant site, acute retention, urinary tract infection, hematuria, de novo urge incontinence, dysuria, urinary urgency, vaginal infection/irritation/Lichen Sclerosus, and worsening urinary incontinence.
Caution: U.S. Federal law restricts this device to sale and use by, or on the order of, a physician.
For a complete listing of indications, contraindications, warnings and precautions, go to www.bulkamid.com/isi.