Axonics aspires to be the global leader in incontinence therapies by providing customer-centric solutions to improve the quality of life for patients suffering from urinary and bowel dysfunction.



Axonics® provides your patients a long-lived, easy-to-use, sacral neuromodulation system, featuring:

Easy-to-use pocket-sized patient Remote Control that does not require charging.
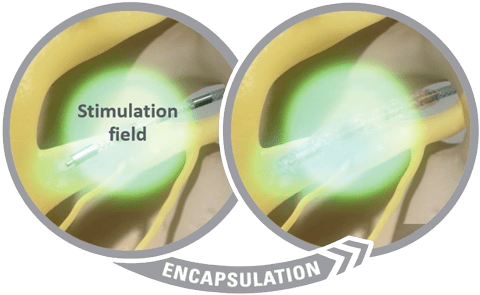
The Axonics SNM System delivers constant current stimulation to help maintain the right amount of therapy and reduce the need for patients to make adjustments.1
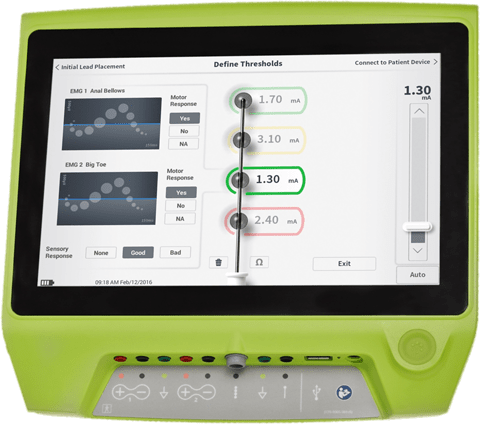
User-friendly interface and proprietary algorithm that generates recommendations based on intraoperative responses and lead placement.


My quality of life is still absolutely brilliant six years after receiving the implant.
Results and experiences are unique to each patient and may vary.
 Advantage
Advantage

Homogenous hydrogel consisting of 97.5% water and 2.5% cross-linked polyacrylamide2.
Allows for reproducible placement of the Bulkamid cushions under direct vision.
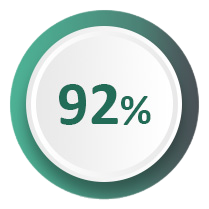
92% of women report being cured or improved at 12 months2, with durability demonstrated at seven years3 and no serious adverse events4.

I am ecstatic with my results. I can now be active with my daughter and not worry about leaking in my pants.
Results and experiences are unique to each patient and may vary.
Provide your information below to be contacted by the Axonics Representative near you.

Indications:
Axonics SNM Therapy for urinary control is indicated for the treatment of urinary retention and the symptoms of overactive bladder, including urinary urge incontinence (leakage) and significant symptoms of urgency-frequency, either alone or in combination, in patients who have failed or could not tolerate more conservative treatments. Axonics SNM Therapy for bowel control is indicated for the treatment of chronic faecal incontinence. The therapy should only be used in patients who have failed or are not candidates for more conservative treatments.
Contraindications:
The Axonics SNM System is contraindicated for patients who have not demonstrated an appropriate response to test stimulation or patients who are unable to operate the Axonics SNM System.
Warnings:
This therapy is not intended for patients with mechanical obstruction such as benign prostatic hypertrophy, cancer, or urethral stricture.
Implantation and use of the Axonics System incurs risk beyond those normally associated with surgery, some of which may necessitate surgical intervention. These risks include but are not limited to adverse change in voiding function (bowel and/or bladder), infection, pain or irritation at the implant site, lead or device migration, electrical shock, change in sensation or magnitude of stimulation which has been described as uncomfortable (jolting or shocking) by some patients, and heating or burns at the device site.
Results and experiences may vary and are unique to each patient. No promise or guarantee is made about specific results or experiences. Talk to your doctor about whether the Axonics System is right for you and to discuss the potential risks and benefits.
For more information about safety and potential risks, refer to Information for Prescribers and Patients at www.axonics.com/eIFU.
Precautions:
The safety and effectiveness of the Axonics System has not been established for use in women who are pregnant or in delivery; for pediatric patients (under the age of 18 years for faecal incontinence and under the age of 16 years for overactive bladder and urinary retention); for patients with neurological disease origins, such as multiple sclerosis or diabetes; or for bilateral stimulation.
Caution:
This device can be sold and used by, or on the order of, a physician only. For Summary of Safety and Clinical Performance (SSCP), refer to https://ec.europa.eu/tools/eudamed/#/screen/home.
Adverse Event Reporting
In case of any serious incident related to the product, please report to Axonics (+1-866-717-2190) as well as the competent authority of your state, which can be found here: https://www.ema.europa.eu/en/partners-networks/eu-partners/eu-member-states/national-competent-authorities-human.

Indications:
Bulkamid® Hydrogel is intended to be used as a urethral bulking agent for the treatment of female urinary incontinence where the stress component is significant. Bulkamid® Hydrogel is intended for female patients, above the age of 18 years that have failed conservative treatments.
Intended users:
Bulkamid® Uretheral Bulking System is intended for use by qualified physicians, e.g. gynaecologist, urologist, or urogynaecologist.
Contraindications:
Bulkamid® Urethral Bulking System is a contraindicated in patients suffering from acute cystitis or urethritis.
Do not use in patients who have active Herpes Genitalis.
Do not use Bulkamid® Urethral Bulking System in patients with damaged tissue in the urethra.
Warnings:
Do not inject Bulkamid® Hydrogel intravascularly. It is possible that accidental vascular injection will cause embolism. During the bulking procedure, the blood vessels must always remain visible at the site of injection to avoid the risk of necrosis. Evaluate the condition of the tissue (e.g. hardness, oedema, haematoma, atrophy) at the site of injection prior to treatment. Patients receiving treatment interfering with blood coagulation have an increased risk of haematoma or urethral bleeding.
Precautions:
Do not inject Bulkamid® Hydrogel into sites previously injected with other bulking agents or vice versa. If the patient has undergone major dental work or surgery, Bulkamid® Hydrogel should not be injected until the patient is fully recovered. If the patient needs surgery or major dental work post Bulkamid® Hydrogel injection, antibiotic treatment to reduce risk of infection should be considered by physician. Patients with acute or chronic infection in other sites of the body must be treated with caution. Only patients with well-controlled diabetes should be considered for Bulkamid® Hydrogel injection. Do not inject Bulkamid® Hydrogel into other sites of the body. The procedure may cause urinary tract infections and scratches in urethra and bladder. Prophylactic antibiotic is recommended. It is possible that inflammatory changes seen at the site of implant may later be misinterpreted for other pathology. Do not mix Bulkamid® Hydrogel with any other substances. Discard any unused material/product per local protocol/procedure. Bulkamid® Hydrogel should be used with caution in patients on immunosuppressive therapy. Safety has not been established for patients with autoimmune diseases. Do not introduce Bulkamid Rotatable Sheath into other body cavities. All components of the Bulkamid® Urethral Bulking System are only intended for single patient use and single use. Do not re-use. Reuse increases the risk of contamination and hereby increases the risk of infection. Do not re-sterilize Bulkamid® Hydrogel, Bulkamid® Needle or Bulkamid Rotatable Sheath. Do not use any component of the Bulkamid® Urethral Bulking System components after the expiry date printed on the packaging. The effect of Bulkamid® Hydrogel has not been evaluated in women during pregnancy, delivery, or lactation. A change to the device insertion technique can lead to implant complications.
Adverse Events:
General side effects normally associated with any surgical implantation procedure or local anaesthesia also apply to the placement of Bulkamid® Hydrogel.
Specifically, the following side effects may be associated with the use of the device system: